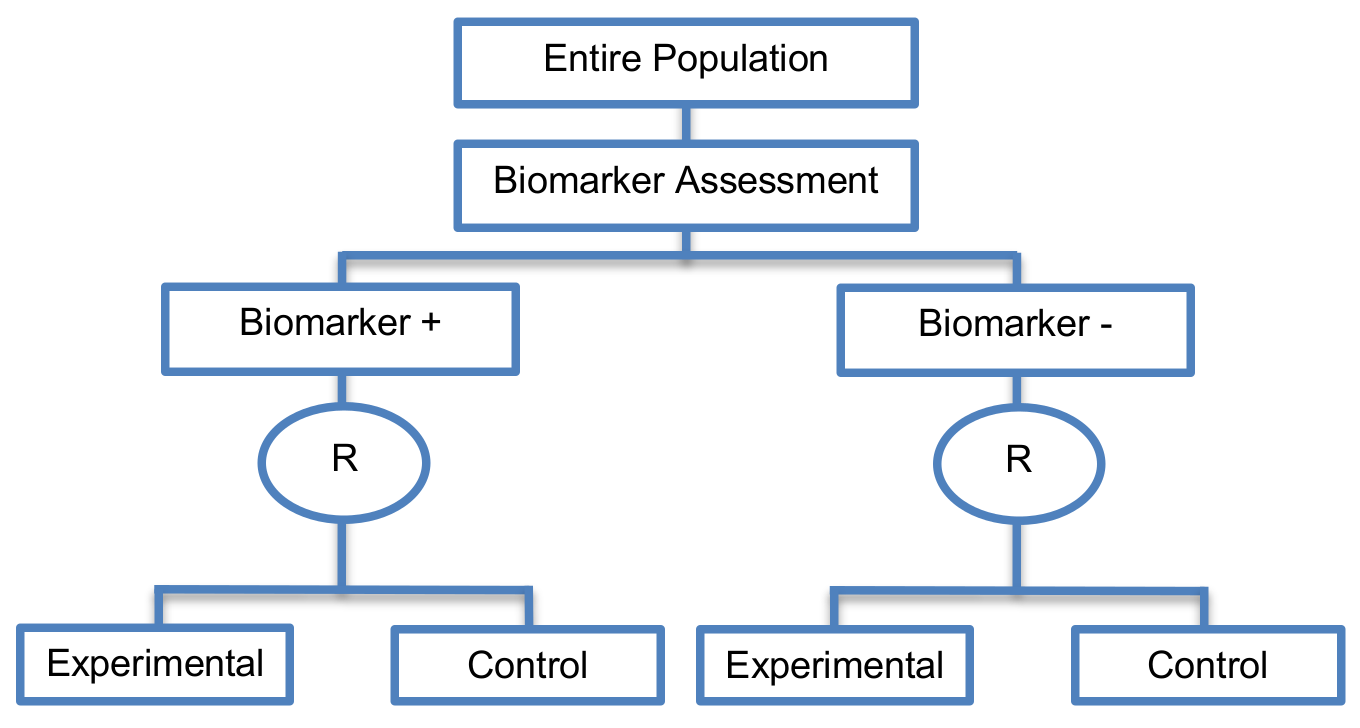
Prospective validation Phase III trials.
Alternative names: Marker-stratified designs, Biomarker-stratified designs, Stratified-Randomized designs, Stratification designs, Stratified designs, Stratified Analysis designs, Marker by treatment – interaction designs, Marker-by-treatment interaction designs, Treatment by marker interaction designs, Treatment-by-marker interaction designs, Marker-treatment interaction designs, Treatment-marker interaction designs, Biomarker-by-treatment interaction designs, Non-targeted RCT (stratified by marker) designs, Genomic Signature stratified designs, Signature-Stratified designs, Randomization or analysis stratified by biomarker status designs, marker-interaction designs.
Details
Utitlity
When there is enough evidence that the experimental treatment is more effective in the positive biomarker-defined subgroup than in the negative biomarker-defined subgroup but there is no sufficient compelling data that the experimental treatment is of no benefit in biomarker-negative individuals, the marker stratified designs can be used.
Methodology
-
Biomarker status is used to stratify the randomization, rather than to restrict eligibility. Marker-stratified designs can be conducted using two different testing plans; the so-called marker-by-treatment interaction with separate tests and marker-by-treatment interaction with interaction test. Both of these approaches involve conducting two independent clinical trials.
- Marker-by-treatment interaction using separate test: This is also referred to as ‘separate randomization designs’ and ‘separate by treatment interaction designs’. This analysis plan is based on separate superiority tests in each biomarker-defined subgroup in order to detect the treatment efficacy in each subgroup. It is a testing plan which determines whether the novel treatment is superior to the control treatment separately within each biomarker-defined subgroup. Consequently, the hypothesis to be tested, the calculation of the number of patients required for the trial, the estimation of the statistical power of the designs and the randomization procedure of patients to different treatments are independent among the different subgroups. The sample size of the trial should be calculated in such a way so as to yield adequate statistical power when testing whether the experimental treatment is superior to the control treatment separately in the two biomarker-defined subgroups. Hence, this approach is not widely used due to the required large sample size as essentially two separate trials are being conducted. Another limitation of this approach is that when multiple biomarker-defined subgroup and treatments are to be investigated, it is difficult to implement in practice.
- Marker-by-treatment interaction using interaction test: It uses a test for interaction between the biomarker status and treatment assignment. Marker stratified designs which use this testing plan are also referred to in the literature as ‘interaction designs’ or ‘genomic signature stratified designs’. First, a formal statistical test for interaction between biomarker status and treatment assignment is undertaken. If this interaction is not significant, then the study is continued by testing the different treatments overall at a two-sided significance level of 0.05, otherwise, the treatments are compared within each biomarker-defined subpopulation at a two-sided 0.05 significance level (i.e., the same as in the marker-by-treatment interaction designs using separate tests). The sample size for this second testing plan is calculated with reference to the treatment effect in the entire study population. Therefore, it might not provide sufficient power for detecting the treatment effect in each biomarker defined-subgroup individually. More precisely, if the sample size is calculated for the overall analysis and the proportion of the biomarker-defined subpopulation which responds to the novel treatment is very small, the statistical power for the subgroup analysis may be inadequate. In addition, when several biomarker-defined subpopulations and treatments are to be investigated, this strategy is not easy to be implemented.
-
Zhao and Simon have developed an online tool for the calculation of sample size for biomarker stratified randomized designs with binary or time-to-event endpoints which is available online at the following web site http://brb.nci.nih.gov/brb/samplesize/sdpap.html. More precisely, the sample size for both binary and time-to-event endpoints can be performed with three different analysis plans; A, B and C. Before choosing one of these analysis plans on the web site, for binary endpoints we need to specify the probability of treatment response in the control arm as well as the proportion of biomarker-positive patients. For survival endpoints, the hazard ratio of biomarker-positive patients versus the biomarker-negative control patients which corresponds to the hazard ratio of prognostic effect as well as the proportion of biomarker-positive patients must be specified.
- Analysis plan A: It is performed when there is confidence that an overall treatment effect exists. It determines the sample size on the basis of first of all comparing the experimental treatment to the control treatment in the entire randomized population at a reduced two-sided significance level α<.05. If the overall test is not significant, then the experimental treatment is compared to the control treatment in the biomarker-positive patients using the significance level 0.05 - α. Analysis Plan A is similar to the ‘Biomarker-positive and overall strategies designs’ with fall-back analysis; the difference lies in terms of the significance levels they have used. In order for the sample size to be estimated, the anticipated overall effect estimate, reduced two-sided significance level and power for the overall test need to be specified.
- Analysis plan B: It is performed when there is confidence that there is a treatment effect in the biomarker-positive subpopulation. It determines the sample size on the basis of first of all comparing the experimental treatment to the control treatment in the biomarker-positive subgroup at a two-sided significance level of α=0.05 level. If the treatment effect is found to be significant at this 0.05 level, then treatment effect is evaluated in the biomarker-negative subgroup again at a two-sided significance level of 0.05 level. This analysis plan is identical to the ‘Sequential subgroup specific designs’. In order for the sample size to be estimated, apart from the fixed significance level set to 0.05, the anticipated effect estimate in the biomarker-positive subpopulation and power need to be specified.
- Analysis plan C: It first tests whether there is a statistically significant interaction between treatment and biomarker. If the interaction is not significant, then the treatments are compared in the overall study population at a two-sided significance level 0.05. Otherwise, the treatments are compared within the two biomarker subgroups separately at a two-sided 0.05 significance level for each subgroup. Analysis Plan C follows either the ‘marker-by-treatment interaction process with interaction or the separate test process’ . In order for the sample size to be estimated, the anticipated treatment effect in the overall study population, the one-sided significance level for interaction test and the power for testing the treatment effect in the overall population need to be specified.
Sample size formula
-
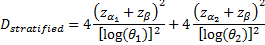
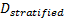 is referred to the required total
number of events for the achievement of sufficient power in each biomarker-defined
subgroup separately (time-to-event endpoint),
is referred to the required total
number of events for the achievement of sufficient power in each biomarker-defined
subgroup separately (time-to-event endpoint),
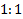 ratio between the two treatment
arms (experimental:control) is assumed,
ratio between the two treatment
arms (experimental:control) is assumed,
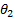 corresponds to the hazard ratio
of biomarker-negative subgroup,
corresponds to the hazard ratio
of biomarker-negative subgroup,
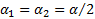 .
.
-
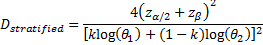
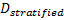 is referred to the required total
number of events for the achievement of sufficient power in the overall
population (time-to-event endpoint),
is referred to the required total
number of events for the achievement of sufficient power in the overall
population (time-to-event endpoint),
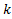 is the proportion biomarker-positive
patients,
is the proportion biomarker-positive
patients,
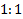 ratio between the two treatment
arms (experimental:control) is assumed.
ratio between the two treatment
arms (experimental:control) is assumed.
-
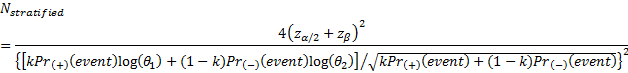
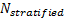 is referred to the required total
number of patients for the achievement of sufficient power in the overall
population (time-to-event endpoint),
is referred to the required total
number of patients for the achievement of sufficient power in the overall
population (time-to-event endpoint),
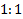 ratio between the two treatment
arms (experimental:control) is assumed,
ratio between the two treatment
arms (experimental:control) is assumed,
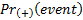 ,
,
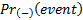 are the probabilities of an event
in biomarker-positive subgroup and biomarker-negative subgroup respectively.
are the probabilities of an event
in biomarker-positive subgroup and biomarker-negative subgroup respectively.
-
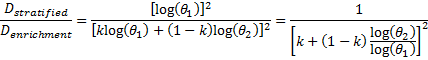
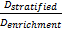 is referred to the ratio of the
required number of events between marker stratified and enrichment designs
(time-to-event endpoint).
is referred to the ratio of the
required number of events between marker stratified and enrichment designs
(time-to-event endpoint).
-
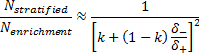
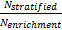 is referred to the ratio of the
required number of patients between marker stratified and enrichment designs
(binary outcome),
is referred to the ratio of the
required number of patients between marker stratified and enrichment designs
(binary outcome),
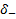 ,
,
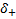 , correspond to the treatment
effectiveness in biomarker-negative and biomarker-positive subgroup
respectively.
, correspond to the treatment
effectiveness in biomarker-negative and biomarker-positive subgroup
respectively.
-
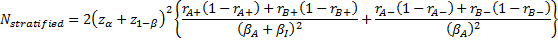
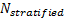 is referred to the required total
number of patients (binary outcome),
is referred to the required total
number of patients (binary outcome),
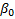 denotes a baseline effect,
denotes a baseline effect,
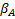 denotes the added effect of the
experimental treatment,
denotes the added effect of the
experimental treatment,
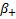 denotes the biomarker-positive
effect and
denotes the biomarker-positive
effect and
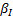 denotes the nonadditive effect,
denotes the nonadditive effect, 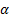 corresponds
to the target level,
corresponds
to the target level,
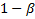 corresponds to the power,
corresponds to the power,
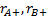 are the assumed response rates of
biomarker-positive patients receiving the experimental and the control
treatment respectively,
are the assumed response rates of
biomarker-positive patients receiving the experimental and the control
treatment respectively,
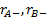 are the assumed response rates of
biomarker-negative patients receiving the experimental and the control
treatment respectively.
are the assumed response rates of
biomarker-negative patients receiving the experimental and the control
treatment respectively.
Statistical/Practical considerations
Advantages
- Ability to assess the treatment effect not only in the entire population but also in each biomarker-defined subgroup. Thus, these designs can find the optimal treatment in the entire population and in each biomarker-defined subgroup.
- Ethical designs even in situations where the biomarker is not useful as no treatment decisions are made based on biomarker status; all decisions are made randomly. Consequently, if the biomarker’s value is in doubt, these designs may be preferred.
Limitations
- In situations where there are several biomarkers and treatments these designs may not be feasible as it involves randomization of patients between all possible treatment options and may require a large sample size.
- May not be feasible when the prevalence of the biomarker is low.
- Might be expensive to test the entire population for its biomarker status.
- Measuring the biomarker up front may be logistically difficult.
- There is no guarantee of balanced groups for analysis.
Included designs
- Subgroup-specific designs (i.e., sequential subgroup-specific designs, parallel subgroup-specific designs)
- Biomarker-positive and overall strategies (i.e., sequential subgroup-specific designs, parallel subgroup-specific designs)
- Marker sequential test designs (MaST) (i.e., sequential subgroup-specific designs, parallel subgroup-specific designs)
Key references
- Shi, Q.; Mandrekar, S.J.; Sargent, D.J. Predictive biomarkers in colorectal cancer: Usage, validation, and design in clinical trials. Scand. J. Gastroenterol. 2012, 47, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Buyse, M.; Michiels, S. Omics-based clinical trial designs. Curr. Opin. Oncol. 2013, 25, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sargent, D.J.; Conley, B.A.; Allegra, C.; Collette, L. Clinical trial designs for predictive marker validation in cancer treatment trials. J. Clin. Oncol. 2005, 23, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lu, T.-P.; Chen, D.-T.; Wang, S.-J. Biomarker adaptive designs in clinical trials. Transl. Cancer Res. 2014, 3, 279–292. [Google Scholar]
- Gosho, M.; Nagashima, K.; Sato, Y. Study designs and statistical analyses for biomarker research. Sensors 2012, 12, 8966–8986. [Google Scholar] [CrossRef] [PubMed]
- Ming-Wen An, S.J.M.; Daniel, J.S. Biomarkers-guided targeted drugs: New clinical trials design and practice necessity. Adv. Personal. Cancer Manag. 2011, 30–41. [Google Scholar]
- Buyse, M. Towards validation of statistically reliable biomarkers. Eur. J. Cancer Suppl. 2007, 5, 89–95. [Google Scholar] [CrossRef]
- Lee, C.K.; Lord, S.J.; Coates, A.S.; Simes, R.J. Molecular biomarkers to individualise treatment: Assessing the evidence. Med. J. Aust. 2009, 190, 631–636. [Google Scholar] [PubMed]
- Simon, R. Clinical trial designs for evaluating the medical utility of prognostic and predictive biomarkers in oncology. Personal. Med. 2010, 7, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, S.J.; Sargent, D.J. Design of clinical trials for biomarker research in oncology. Clin. Investig. 2011, 1, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Beckman, R.A.; Clark, J.; Chen, C. Integrating predictive biomarkers and classifiers into oncology clinical development programmes. Nat. Rev. Drug Discov. 2011, 10, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Young, K.Y.; Laird, A.; Zhou, X.H. The efficiency of clinical trial designs for predictive biomarker validation. Clin. Trials 2010, 7, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Xuemin, G.; Suyu, L. Bayesian adaptive randomization designs for targeted agent development. Clin. Trials 2010, 7, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, S.J.; Sargent, D.J. Clinical trial designs for predictive biomarker validation: Theoretical considerations and practical challenges. J. Clin. Oncol. 2009, 27, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, S.J.; Sargent, D.J. Clinical trial designs for predictive biomarker validation: One size does not fit all. J. Biopharm. Stat. 2009, 19, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Sigman, C.C. Cancer biomarkers: Selecting the right drug for the right patient. Nat. Rev. Drug Discov. 2012, 11, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, S.J.; Sargent, D.J. Predictive biomarker validation in practice: Lessons from real trials. Clin. Trials 2010, 7, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Wu, W.; Sarkaria, J.; Chang, S.M.; Colman, H.; Sargent, D.; Reardon, D.A. Incorporation of biomarker assessment in novel clinical trial designs: Personalizing brain tumor treatments. Curr. Oncol. Rep. 2011, 13, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Van Schaeybroeck, S.; Allen, W.L.; Turkington, R.C.; Johnston, P.G. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat. Rev. Clin. Oncol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Freidlin, B.; Korn, E.L. Biomarker enrichment strategies: Matching trial design to biomarker credentials. Nat. Rev. Clin. Oncol. 2014, 11, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Polley, E. Clinical trials for precision oncology using next-generation sequencing. Personal. Med. 2013, 10, 485–495. [Google Scholar] [CrossRef]
- Baker, S.G.; Kramer, B.S.; Sargent, D.J.; Bonetti, M. Biomarkers, subgroup evaluation, and clinical trial design. Discov. Med. 2012, 13, 187–192. [Google Scholar] [PubMed]
- Simon, R. Clinical trials for predictive medicine. Stat. Med. 2012, 31, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, K.K. Statistical design and evaluation of biomarker studies. Methods Mol. Biol. 2014, 1102, 667–677. [Google Scholar] [PubMed]
- Freidlin, B.; McShane, L.M.; Korn, E.L. Randomized clinical trials with biomarkers: Design issues. J. Natl. Cancer Inst. 2010, 102, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Galanis, E. Incorporation of prognostic and predictive factors into glioma clinical trials. Curr. Oncol. Rep. 2013, 15, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Collette, L.; Bogaerts, J.; Suciu, S.; Fortpied, C.; Gorlia, T.; Coens, C.; Mauer, M.; Hasan, B.; Collette, S.; Ouali, M.; et al. Statistical methodology for personalized medicine: New developments at EORTC headquarters since the turn of the 21st century. Eur. J. Cancer Suppl. 2012, 10, 13. [Google Scholar] [CrossRef]
- Simon, R. Development and validation of biomarker classifiers for treatment selection. J. Stat. Plan. Inference 2008, 138, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Freidlin, B.; McShane, L.M.; Polley, M.-Y.C.; Korn, E.L. Randomized phase II trial designs with biomarkers. J. Clin. Oncol. 2012, 30, 3304–3309. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Koch, A.; Krockenberger, K.; Grosshennig, A. Personalized medicine using DNA biomarkers: A review. Hum. Genet. 2012, 131, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Freidlin, B.; Korn, E.L. Biomarker-adaptive clinical trial designs. Pharmacogenomics 2010, 11, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, J.C.; Kim, K.; Beach, J.; Kolesar, J.M.; Gee, J.R. A bayesian adaptive design with biomarkers for targeted therapies. Clin. Trials 2010, 7, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Scheibler, F.; Zumbé, P.; Janssen, I.; Viebahn, M.; Schröer-Günther, M.; Grosselfinger, R.; Hausner, E.; Sauerland, S.; Lange, S. Randomized controlled trials on pet: A systematic review of topics, design, and quality. J. Nucl. Med. 2012, 53, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- An, M.-W.; Mandrekar, S.J.; Sargent, D.J. A 2-stage phase II design with direct assignment option in stage II for initial marker validation. Clin. Cancer Res. 2012, 18, 4225–4233. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wu, C.O.; Yang, S.; Waclawiw, M.A.; DeMets, D.L.; Geller, N.L. NHLBI clinical trials workshop: An executive summary. Stat. Med. 2012, 31, 2938. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; He, P. Reinventing clinical trials: A review of innovative biomarker trial designs in cancer therapies. Br. Med. Bull. 2015, 114, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Renfro, L.A.; Mallick, H.; An, M.-W.; Sargent, D.J.; Mandrekar, S.J. Clinical trial designs incorporating predictive biomarkers. Cancer Treat. Rev. 2016, 43, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ondra, T.; Dmitrienko, A.; Friede, T.; Graf, A.; Miller, F.; Stallard, N.; Posch, M. Methods for identification and confirmation of targeted subgroups in clinical trials: A systematic review. J. Biopharm. Stat. 2016, 26, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Wang, S.J. Use of genomic signatures in therapeutics development in oncology and other diseases. Pharmacogenom. J. 2006, 6, 166–173. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Reflection Paper on Methodological Issues Associated with Pharmacogenomic Biomarkers in Relation to Clinical Development and Patient Selection. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500108672.pdf (accessed on 10 October 2015).
- Lai, T.L.; Liao, O.Y.-W.; Kim, D.W. Group sequential designs for developing and testing biomarker-guided personalized therapies in comparative effectiveness research. Contemp. Clin. Trials 2013, 36, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N. Analysis of randomized controlled clinical trials. Methods Mol. Biol. 2009, 473, 113–126. [Google Scholar] [PubMed]
- Tajik, P.; Bossuyt, P.M. Genomic markers to tailor treatments: Waiting or initiating? Hum. Genet. 2011, 130, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Eng, K.H. Randomized reverse marker strategy design for prospective biomarker validation. Stat. Med. 2014, 33, 3089–3099. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.G. Biomarker evaluation in randomized trials: Addressing different research questions. Stat. Med. 2014, 33, 4139–4140. [Google Scholar] [CrossRef] [PubMed]